News Archive
 Program of seminarProgram of the joint seminars of UCT Prague and IOCB held usually on Fridays at 2:30 pm in room B2319 or IOCB club for summer term 2024/25
Program of seminarProgram of the joint seminars of UCT Prague and IOCB held usually on Fridays at 2:30 pm in room B2319 or IOCB club for summer term 2024/25 Rudolf Kvasňovský Succeeded with Poster in Hünfeld
Rudolf Kvasňovský Succeeded with Poster in Hünfeld Rudolf Kvasňovský, a third-year student of the bachelor's program Chemistry, won second place in the competition for the best poster at the Hünfeld 2025: Workshop on Computer Simulation and Theory of Macromolecules conference. Congratulations!
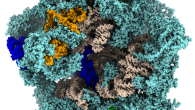
R. Kvasňovský is member of Michal Kolář's research group, where he focuses on simulations of the mitochondrial ribosome. Unlike universal ribosomes, which are found in the cytosol or on the endoplasmic reticulum of eukaryotic cells, the mitochondrial ribosome is highly specialized. In human cells, it synthesizes only 13 proteins, whereas universal ribosomes synthesize several tens of thousands of proteins. R. Kvasňovský's poster, titled Simulation Model of the Mitochondrial Ribosome, represents nearly a year of work.
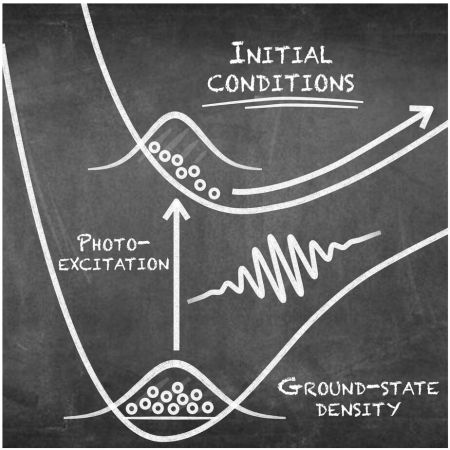 Article in Accounts of Chemical Research
Article in Accounts of Chemical ResearchPetr Slavíček and Jiří Janoš together with Basile Curchod from Bristol University discuss the limits of trajectory-based approaches for photochemical simulations: https://pubs.acs.org/doi/10.1021/acs.accounts.4c00687
 DefenceOn Dec 02, 2024, Martin Klíma defended PhD thesis “Nucleation during supersonic expansion to vacuum”. Congratulations!
DefenceOn Dec 02, 2024, Martin Klíma defended PhD thesis “Nucleation during supersonic expansion to vacuum”. Congratulations! Daniel Bím received a prestigious GAČR Junior Star grant
Daniel Bím received a prestigious GAČR Junior Star grantThe funded project focuses on developing advanced nickel catalysts for efficient use in redox and photochemical reactions as an environmentally friendly alternative to methods that rely on precious and toxic metals.
 DefenceOn Sep 17, 2024, Jiří Janek defended PhD thesis “Time-reversible predictors in molecular dynamics”
DefenceOn Sep 17, 2024, Jiří Janek defended PhD thesis “Time-reversible predictors in molecular dynamics”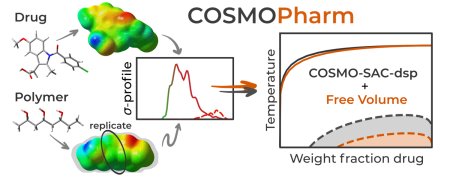 COSMOPharm
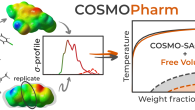
COSMOPharmThe Laboratory of Computational Thermodynamics, in collaboration with Technische Universität Berlin, presents in a new study published in Molecular Pharmaceutics the capabilities of the quantum mechanics-aided COSMO-SAC model to predict the compatibility of drugs and polymers. This is a key aspect in the design of drug formulations based on amorphous solid dispersions. The related computational tool COSMOPharm is freely available on GitHub. The study was supported by the Czech Science Foundation (project 22-07164S).
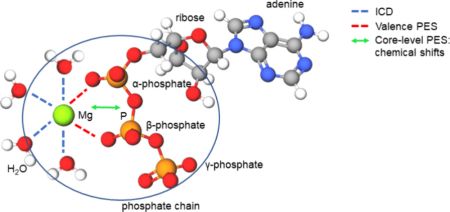 New paper in JACS
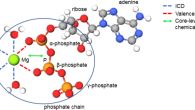
New paper in JACSA new work “How Does Mg2+(aq) Interact with ATP(aq)? Biomolecular Structure through the Lens of Liquid-Jet Photoemission Spectroscopy” was published Journal of the Amercial Chemical Society. The research team PHOTOX from our department has provided the interpretation of the experimental results.
The Laboratory of computational thermodynamics offers the following positions:
 Successful Student Professional Activity
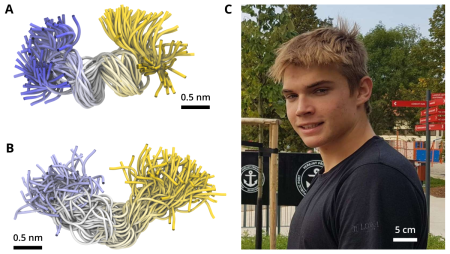
Successful Student Professional ActivityFrom June 16th to 18th, 2023, the 45th National Exhibition of Student Professional Activities (aka SOČ) took place in Plzeň. Jiří Kubíček, a student from Gymnázium Nad Kavalírkou in Prague, working in the Laboratory of Biomolecular Dynamics at our institute, placed 4th in the Chemistry section with a project titled "Investigation of the Helicity of C-Terminal Fragments of Peptide Deformylases." Congratulations!
Jiří is a third-year gymnasium student who enjoys swimming with fins. Besides sports, he cultivates an interest in chemistry and natural sciences. He met the head of the laboratory, doc. Kolář, during the Summer School for Young Chemists and Biologists at Běstvina and shortly after that, he started studying the details of bacterial proteosynthesis.
Figure: C-terminal PDF segment with high helicity (A) or negligible helicity (B), and the author of the study, Jiří Kubíček (C).
Protein synthesis occurs in a similar manner in all organisms, but there are certain differences. One of them is the presence of a peptide deformylase (PDF) in bacteria, which is absent in higher organisms. PDF's role is to chemically modify the beginning of each protein produced by bacteria, and thus, it represents one of the targets for antibiotic therapy. PDF binds to the ribosome, the cellular protein factory, through its C-terminal part. It turns out that different bacteria have PDFs with different C-terminal segments. Some are capable of forming an α-helix, thus binding to the ribosome, while it is not known how others, which probably do not form α-helices, bind to the ribosome. Jiří Kubíček's research focused on the C-terminal segment.
He performed molecular dynamics computer simulations to elucidate the propensity of various PDFs to form α-helices, so-called helicity. Out of the five studied C-terminal segments, only one exhibited high helicity. The other segments behaved comparably in terms of helicity and formed almost no α-helices. Knowledge gained during the study sheds light on the binding of non-helical PDF C-termini on the ribosome. This may, in the long term, help discover new strategies to fight bacterial infections.
SOČ is a multidisciplinary competition held since 1979 for high school students interested in science. During the school, district, regional, and national rounds of the competition, participants have to defend their scientific projects before expert committees, which they usually develop in collaboration with universities or scientific institutions. Thus, participants have the opportunity to experience real research, including literature review, obtaining results, and their critical evaluation, while still in high school. The best participants then represent the Czech Republic in international competitions of a similar format.
 Protective osmolytes are depleted from protein surface
Protective osmolytes are depleted from protein surfaceTheoretical (computational) modelling of complex protein-osmolyte interactions has always been challenging. Nowadays, computational power allows us to obtain numerically exact results for small to mid-size protein denaturation. But, are the current parameterizations of protein-osmolyte interactions reliable? This is difficult to answer without accurate experimental data to which we can benchmark our simulations. Our recent work in Journal of Physical Chemistry Letters, contributed to this important question on thermodynamic means.
We applied dialysis experiments on lysozyme protein and circular-dichroism on TrpCage miniprotein and quantified the depletion of protective osmolytes (TMAO, betaine) from protein surface.
On the computational side, we employed numerous parameterization of TMAO and betaine, which accurately reproduce experimental properties of aqueous solutions. Surprisingly, we have found that impact of stabilizing osmolytes on protein introduced in the solution can be very diverse. We could vaguely say, this stems from our belief that force-fields which perform well piece-wise, will perform well also when combined together. However, without careful readjustment of weak protein-osmolytes interactions, the simulation results can be even qualitatively wrong (i.e., observing protein denaturation).
This important theoretical message along with the urgent need for solid experimental data are presented in our concise letter, which is supported by an extensive SI :)
Invitation to seminar on March 31, 2021 at 2 pm of the Department of Physical Chemistry, University of Chemistry and Technology Prague where Dr. Chhabilal Regmi (postdoc ChemJets2 at UCT) will present a lecture Advanced Bifunctional Materials.
How volatile ionic liquids actually are?
Contact: Ing. Ctirad Červinka, Ph.D., more information
Ionic liquids (ILs) possess a vast potential for numerous technologies (gas capture, smart electrolytes, nanoparticles exfoliation media, etc.), as ILs possess unique properties such as low volatility, large electrochemical window or a boundless structural variability. Broader exploitation of their beneficial characteristics is impeded by their cost, limited availability of the physico-chemical data or an insufficient understanding of ILs-related phenomena. Known for a century and considered nonvolatile for decades, ILs were proved to possess a non-zero saturated vapor pressure only a decade ago. Still, volatility of ILs is lower by orders of magnitude when compared to common molecular solvents. Such an extremely low volatility is a principal obstacle impeding reliable and reproducible measurements of their vaporization data. Significance of these properties for extremely low-volatile chemical species can be illustrated by the environmental distribution of highly persistent compounds, considered almost non-volatile. These, however, spread via the atmosphere even to the polar regions, where they concentrate in plants, animal or human tissues, acting as mutagens subsequently. Data on volatility of chemical species are the key inputs for modelling the environmental distribution of pollutants.
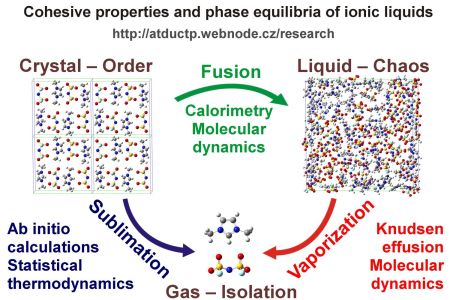
Fig: Schematic representation of computational and experimental techniques planned to be used for individual phases of ILs.
A rigorous methodology, capable of accurate determinations of the volatility (and other phase equilibria) of ILs, which would be based on more reliable approaches (combining experiments and first-principles calculations) than the direct measurements of vapor pressures of ILs would certainly find use in the future process design and selection of suitable ILs. Especially if there are millions of relevant ILs systems, while the thermodynamic data exist for hundreds of ILs. Employment of high-level ab initio calculations, molecular dynamics and experimental calorimetry or effusion measurements represents a worldwide unique approach for solving such a task.
Ribosome peptide tunnel
Contact: RNDr. Michal H. Kolář, Ph.D., http://mhko.science
Proteins are key biomolecules which participate in almost all processes in cells. In living organisms, the proteins are synthesized by large biomolecular complexes called ribosomes. Ribosomes read the genetic information temporarily stored in a strand of ribonucleic acid, and translate it into a sequence of amino acids. The ribosomes catalyze the formation of peptide bonds making it possible under conditions common in living matter.
Because the catalytic center is buried deep in the ribosome, all nascent proteins leave the ribosome through an exit tunnel. The tunnel has important medicinal implications. It contains a binding site for a large class of antibiotics and incorrect protein folding initiated near the tunnel exit may lead to severe neurodegenerative disorders.
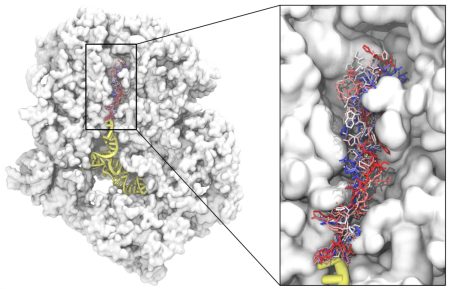
Fig: A cross-section through a prokaryotic ribosome with a several conformations of a nascent protein in the exit tunnel.
Hence, the project will focus on what happens inside the exit tunnel. Such information is difficult to obtain by traditional experimental techniques like X-ray crystallography or cryo-electron microscopy, because the tunnel content is rather flexible, hence blurred on the images. Large-scale molecular dynamics simulations offer an alternative with high spatial and temporal resolution. Using such simulations, Michal and his coworkers will study, how the tunnel content affects the dynamics of the ribosome. They hope to identify new pathways which transfer a signal from the ribosome interior towards its surface.
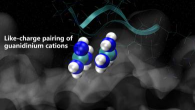
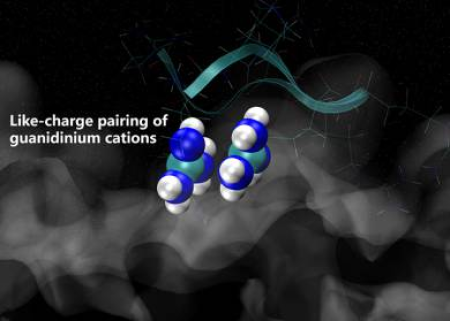 Arginine "magic" of guanidinium cation!
Arginine "magic" of guanidinium cation!Arginine magic in Accounts of Chemical Research
We knew that guanidinium cation is an unique species, which desire our attention. Its ability to form cation-cation pairs is manifested in aqueous solutions of guanidinium salts as well as between arginine sidechains in proteins.
Such arginine magic was pointed out to lead to an unexpected attraction between polyarginine chains with consequences to the action of arginine-rich cell penetrating peptides.
For more, check our recent review in Accounts of Chemical Research.
doi: 10.1021/acs.accounts.8b00098
 ChemJetsApply for full-time two-year junior researcher contracts with possibility of extension. Deadline for application submission November 27, 2017.
ChemJetsApply for full-time two-year junior researcher contracts with possibility of extension. Deadline for application submission November 27, 2017.test