stdClass Object
(
[nazev] => Department of Physical Chemistry
[adresa_url] =>
[api_hash] =>
[seo_desc] =>
[jazyk] =>
[jednojazycny] =>
[barva] =>
[indexace] => 1
[obrazek] =>
[ga_force] =>
[cookie_force] =>
[secureredirect] =>
[google_verification] => UOa3DCAUaJJ2C3MuUhI9eR1T9ZNzenZfHPQN4wupOE8
[ga_account] => UA-10822215-3
[ga_domain] =>
[ga4_account] => G-VKDBFLKL51
[gtm_id] =>
[gt_code] =>
[kontrola_pred] =>
[omezeni] => 0
[pozadi1] =>
[pozadi2] =>
[pozadi3] =>
[pozadi4] =>
[pozadi5] =>
[robots] =>
[htmlheaders] =>
[newurl_domain] => 'ufch.vscht.cz'
[newurl_jazyk] => 'en'
[newurl_akce] => '[en]'
[newurl_iduzel] =>
[newurl_path] => 8548/11349/11352
[newurl_path_link] => Odkaz na newurlCMS
[iduzel] => 11352
[platne_od] => 06.05.2024 10:31:00
[zmeneno_cas] => 06.05.2024 10:31:49.925472
[zmeneno_uzivatel_jmeno] => Martin Klajmon
[canonical_url] =>
[idvazba] => 13420
[cms_time] => 1752877962
[skupina_www] => Array
(
)
[slovnik] => stdClass Object
(
[logo_href] => /
[logo] =>  [google_search] => 001523547858480163194:u-cbn29rzve
[social_fb_odkaz] =>
[social_tw_odkaz] =>
[social_yt_odkaz] =>
[paticka_mapa_alt] =>
[paticka_budova_a_nadpis] => BUILDING A
[paticka_budova_a_popis] => Rector,
Department of Communications,
Department of Education,
FCT Dean’s Office,
Centre for Information Services
[paticka_budova_b_nadpis] => BUILDING B
[paticka_budova_b_popis] => Department of R&D, Dean’s Offices:
FET,
FFBT,
FCE,
Computer Centre,
Department of International Relations,
Bursar
[paticka_budova_c_nadpis] => BUILDING C
[paticka_budova_c_popis] => Crèche Zkumavka,
General Practitioner,
Department of Economics and Management,
Department of Mathematics
[paticka_budova_1_nadpis] => NATIONAL LIBRARY OF TECHNOLOGY
[paticka_budova_1_popis] =>
[paticka_budova_2_nadpis] => CAFÉ CARBON
[paticka_budova_2_popis] =>
[paticka_adresa] => UCT Prague
[google_search] => 001523547858480163194:u-cbn29rzve
[social_fb_odkaz] =>
[social_tw_odkaz] =>
[social_yt_odkaz] =>
[paticka_mapa_alt] =>
[paticka_budova_a_nadpis] => BUILDING A
[paticka_budova_a_popis] => Rector,
Department of Communications,
Department of Education,
FCT Dean’s Office,
Centre for Information Services
[paticka_budova_b_nadpis] => BUILDING B
[paticka_budova_b_popis] => Department of R&D, Dean’s Offices:
FET,
FFBT,
FCE,
Computer Centre,
Department of International Relations,
Bursar
[paticka_budova_c_nadpis] => BUILDING C
[paticka_budova_c_popis] => Crèche Zkumavka,
General Practitioner,
Department of Economics and Management,
Department of Mathematics
[paticka_budova_1_nadpis] => NATIONAL LIBRARY OF TECHNOLOGY
[paticka_budova_1_popis] =>
[paticka_budova_2_nadpis] => CAFÉ CARBON
[paticka_budova_2_popis] =>
[paticka_adresa] => UCT Prague
Technická 5
166 28 Prague 6 – Dejvice
IČO: 60461373 / VAT: CZ60461373
Czech Post certified digital mail code: sp4j9ch
Copyright: UCT Prague 2015
Information provided by the Department of International Relations and the Department of R&D. Technical support by the Computing Centre.
[paticka_odkaz_mail] => mailto:Martin.Klajmon@vscht.cz
[social_fb_title] =>
[social_tw_title] =>
[social_yt_title] =>
[drobecky] => You are here: UCT Prague – FCHIUFCH
[aktualizovano] => Updated
[autor] => Author
[intranet_odkaz] => http://intranet.vscht.cz/
[intranet_text] => Intranet
[more_info] => more info
[den_kratky_2] => Tue
[novinky_kategorie_1] => UCT Events
[novinky_kategorie_2] => Important Dates
[novinky_kategorie_3] => Student Events
[novinky_kategorie_4] => Fun
[novinky_kategorie_5] => Science
[novinky_archiv_url] => /news
[novinky_servis_archiv_rok] => Annual Archive
[novinky_servis_nadpis] => News Settings
[novinky_dalsi] => More News
[logo_mobile_href] => /
[logo_mobile] =>  [mobile_over_nadpis_menu] => Menu
[mobile_over_nadpis_search] => Search
[mobile_over_nadpis_jazyky] => Languages
[mobile_over_nadpis_login] => Login
[menu_home] => Homepage
[zobraz_desktop_verzi] => switch to desktop version
[novinky_archiv] => News Archive
[zobraz_mobilni_verzi] => switch to mobile version
[archiv_novinek] => News Archive
[paticka_mapa_odkaz] =>
[nepodporovany_prohlizec] => For full access, please use different browser.
[den_kratky_5] => Fri
[preloader] => Wait a second...
[hledani_nadpis] => hledání
[hledani_nenalezeno] => Nenalezeno...
[hledani_vyhledat_google] => vyhledat pomocí Google
[den_kratky_3] =>
[den_kratky_1] =>
[den_kratky_4] =>
[social_in_odkaz] =>
[social_li_odkaz] =>
[den_kratky_0] =>
)
[poduzel] => stdClass Object
(
[11512] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[11517] => stdClass Object
(
[obsah] =>
[iduzel] => 11517
[canonical_url] => //ufch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[11514] => stdClass Object
(
[obsah] =>
[iduzel] => 11514
[canonical_url] => //ufch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[11516] => stdClass Object
(
[obsah] =>
[iduzel] => 11516
[canonical_url] => //ufch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
)
[iduzel] => 11512
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[11513] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[11515] => stdClass Object
(
[nazev] => Department of Physical Chemistry
[seo_title] => Department of Physical Chemistry
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] =>
[mobile_over_nadpis_menu] => Menu
[mobile_over_nadpis_search] => Search
[mobile_over_nadpis_jazyky] => Languages
[mobile_over_nadpis_login] => Login
[menu_home] => Homepage
[zobraz_desktop_verzi] => switch to desktop version
[novinky_archiv] => News Archive
[zobraz_mobilni_verzi] => switch to mobile version
[archiv_novinek] => News Archive
[paticka_mapa_odkaz] =>
[nepodporovany_prohlizec] => For full access, please use different browser.
[den_kratky_5] => Fri
[preloader] => Wait a second...
[hledani_nadpis] => hledání
[hledani_nenalezeno] => Nenalezeno...
[hledani_vyhledat_google] => vyhledat pomocí Google
[den_kratky_3] =>
[den_kratky_1] =>
[den_kratky_4] =>
[social_in_odkaz] =>
[social_li_odkaz] =>
[den_kratky_0] =>
)
[poduzel] => stdClass Object
(
[11512] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[11517] => stdClass Object
(
[obsah] =>
[iduzel] => 11517
[canonical_url] => //ufch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[11514] => stdClass Object
(
[obsah] =>
[iduzel] => 11514
[canonical_url] => //ufch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[11516] => stdClass Object
(
[obsah] =>
[iduzel] => 11516
[canonical_url] => //ufch.vscht.cz
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
)
[iduzel] => 11512
[canonical_url] =>
[skupina_www] => Array
(
)
[url] =>
[sablona] => stdClass Object
(
[class] =>
[html] =>
[css] =>
[js] =>
[autonomni] =>
)
)
[11513] => stdClass Object
(
[obsah] =>
[poduzel] => stdClass Object
(
[11515] => stdClass Object
(
[nazev] => Department of Physical Chemistry
[seo_title] => Department of Physical Chemistry
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] => Head of the Department
Prof. RNDr. et Bc. Petr Slavíček, Ph.D.
e +420 22044 3687
b Petr.Slavicek@vscht.cz

Secretary
Lucie Fialková
e +420 22044 4269
b Lucie.Fialkova@vscht.cz
[urlnadstranka] =>
[ogobrazek] =>
[pozadi] =>
[iduzel] => 11515
[canonical_url] =>
[skupina_www] => Array
(
)
[url] => /home
[sablona] => stdClass Object
(
[class] => stranka_novinky
[html] =>
[css] =>
[js] =>
[autonomni] => 1
)
)
[14703] => stdClass Object
(
[nazev] => About Department of Physical Chemistry
[seo_title] => About Department of Physical Chemistry
[seo_desc] =>
[autor] =>
[autor_email] =>
[perex] => Our department is a part of Faculty of Chemical Engineering of Prague University of Chemistry and Technology. It provides a two-semester course in basic physical chemistry and two-semester tuition in laboratory practice. The aim of the course is to provide students with theoretical background for effective control of important chemical operations, for production of organic and inorganic chemicals, pharmaceutical and petrochemical products.
[ikona] => graf-spojnicovy
[obrazek] =>
[obsah] => In addition to this, in the following years the department provides advanced education in physical chemistry for selected students within the framework of its own specialization. These special courses are designed with emphasis on the research carried out in the department. The graduate will be qualified for a position of a manager or researcher mainly in chemical, petrochemical or pharmaceutical industry; however, this education qualifies him for a variety of other activities, too. For more information see the education.
Of course, the department provides the research work in the field of physical chemistry. The main goal is the applied thermodynamics, but we also study kinetic processes, membrane separations and deal with theoretical physical chemistry. For details see the research activities.
[iduzel] => 14703
[canonical_url] => //ufch.vscht.cz/department
[skupina_www] => Array
(
)
[url] => /department
[sablona] => stdClass Object
(
[class] => stranka_submenu
[html] =>
[css] =>
[js] =>
[autonomni] => 1
)
)
[14704] => stdClass Object
(
[nazev] => People
[seo_title] => People
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] =>
[urlnadstranka] =>
[ogobrazek] =>
[pozadi] =>
[iduzel] => 14704
[canonical_url] =>
[skupina_www] => Array
(
)
[url] => /people
[sablona] => stdClass Object
(
[class] => boxy
[html] =>
[css] =>
[js] => $(function() {
setInterval(function () { $('*[data-countdown]').each(function() { CountDownIt('#'+$(this).attr("id")); }); },1000);
setInterval(function () { $('.homebox_slider:not(.stop)').each(function () { slide($(this),true); }); },5000);
});
function CountDownIt(selector) {
var el=$(selector);foo = new Date;
var unixtime = el.attr('data-countdown')*1-parseInt(foo.getTime() / 1000); if(unixtime<0) unixtime=0;
var dnu = 1*parseInt(unixtime / (3600*24)); unixtime=unixtime-(dnu*(3600*24));
var hodin = 1*parseInt(unixtime / (3600)); unixtime=unixtime-(hodin*(3600));
var minut = 1*parseInt(unixtime / (60)); unixtime=unixtime-(minut*(60));
if(unixtime<10) {unixtime='0'+unixtime;}
if(dnu<10) {unixtime='0'+dnu;}
if(hodin<10) {unixtime='0'+hodin;}
if(minut<10) {unixtime='0'+minut;}
el.html(dnu+':'+hodin+':'+minut+':'+unixtime);
}
function slide(el,vlevo) {
if(el.length<1) return false; var leva=el.find('.content').position().left; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1;
var cislo=leva/sirka*-1; if(vlevo) { if(cislo+1>pocet) cislo=0; else cislo++; } else { if(cislo==0) cislo=pocet-1; else cislo--; }
el.find('.content').animate({'left':-1*cislo*sirka});
el.find('.slider_puntiky a').removeClass('selected');
el.find('.slider_puntiky a.puntik'+cislo).addClass('selected');
return false;
}
function slideTo(el,cislo) {
if(el.length<1) return false; var sirka=el.width(); var pocet=el.find('.content .homebox').length-1; if(cislo<0 || cislo>pocet) return false;
el.find('.content').animate({'left':-1*cislo*sirka});
el.find('.slider_puntiky a').removeClass('selected');
el.find('.slider_puntiky a.puntik'+cislo).addClass('selected');
return false;
}
[autonomni] => 1
)
)
[14708] => stdClass Object
(
[nazev] => Studies
[seo_title] => Studies
[seo_desc] =>
[autor] =>
[autor_email] =>
[obsah] => Our students can find a lot of useful information for the basic physical chemistry and special courses on our web pages.
Textbooks in English
Some of our textbooks are available also in English
- Physical Chemistry in Brief is an English (v print or online version) translation of our popular handbook
- State Behaviour of Liquids – Instructions for laboratory exercises (Cibulka et al.)
Lectures in English
- Molecular modeling and simulation (Kolafa)
- Physical and Colloid Chemistry (Kolafa, Bartovská)
- Mathematical metods for physical chemistry (N403045) (Kolafa, Malijevský)
- Physical Chemistry I (Dohnal, Cibulka)
- Problems (zip, v )
- Texts (thermodyn. of mixtures, VLE, Galvanic cells, zip/pdf v)
- Specimen tests (first, second, third, final exam, pdf v )
- Additional info (pdf, pdf, pdf, v )
Software
Software developed in our department includes both educational and scientific applications. It covers computer simulations, databases, and phase equilibria. More info on separate site.
[urlnadstranka] => [ogobrazek] => [pozadi] => [iduzel] => 14708 [canonical_url] => [skupina_www] => Array ( ) [url] => /studies [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [14706] => stdClass Object ( [nazev] => Seminars of Department of Physical Chemistry [seo_title] => Seminars of Dept. Phys. Chem. [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => mikrofon [obrazek] => [ogobrazek] => [pozadi] => [obsah] =>The joint seminars of Department of Physical Chemistry, University of Chemistry and Technology Prague and Institute of Organic Chemistry and Biochemistry (IOCB), Czech Academy of Sciences, will be held on Fridays, at 2:30 pm in the conference room of Faculty of Chemical Engineering (4th floor, building A of UCT, room A402) or seminar room of IOCB (4th floor, building B "Květák, room B.4.29).
Upcoming talks in spring term 2023/24
[urlnadstranka] => [iduzel] => 14706 [canonical_url] => [skupina_www] => Array ( ) [url] => /seminar [sablona] => stdClass Object ( [class] => stranka_ikona [html] => [css] => [js] => [autonomni] => 1 ) ) [14707] => stdClass Object ( [nazev] => Experiments [seo_title] => Experiments [seo_desc] => [autor] => [autor_email] => [obsah] => [iduzel] => 14707 [canonical_url] => //ufch.vscht.cz/experiments [skupina_www] => Array ( ) [url] => /experiments [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [14705] => stdClass Object ( [nazev] => Research & Development [seo_title] => R & D [seo_desc] => [autor] => [autor_email] => [obsah] => [urlnadstranka] => [ogobrazek] => [pozadi] => [iduzel] => 14705 [canonical_url] => [skupina_www] => Array ( ) [url] => /research [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [24134] => stdClass Object ( [obsah] => [iduzel] => 24134 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => [html] => [css] => [js] => [autonomni] => ) ) ) [iduzel] => 11513 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => [html] => [css] => [js] => [autonomni] => ) ) ) [sablona] => stdClass Object ( [class] => web [html] => [css] => [js] => [autonomni] => 1 ) [api_suffix] => )DATA
stdClass Object
(
[nazev] => Research groups
[seo_title] => Research groups
[seo_desc] =>
[autor] =>
[autor_email] =>
[perex] =>
[ikona] => mikroskop
[obrazek] =>
[vyska] =>
[ogobrazek] =>
[pozadi] =>
[obsah] => Theoretical chemistry
- Statistical thermodynamics and molecular simulations
- Laboratory of computational thermodynamics
- Computer simulations of complex systems
- Division of interfacial phenomena
- Laboratory of theoretical photodynamics
- Division of advanced nanospectroscopic techniques
- Biomolecular dynamics research group
- Laboratory of theoretical spectroscopy
Phase, interphase and chemical equilibria
- Laboratory of experimental thermodynamics: Phase equilibria and properties of mixtures
- Laboratory of phase equilibria: Measurement and prediction of solubilities
- Surface and interface phenomena
- Laboratory of chemical equilibria in solution
Physicochemical properties
- State behavior of aqueous solutions
- Applied thermodynamics
- Laboratory of membrane separation processes
- Prediction of physicochemical properties of pure compounds and mixtures
- Laboratory of photoredox catalysis
Chemical physics
[submenuno] => [drobeckyno] => [urlnadstranka] => [newurl_domain] => 'ufch.vscht.cz' [newurl_jazyk] => 'en' [newurl_akce] => '/research/researchgroups' [newurl_iduzel] => 18574 [newurl_path] => 8548/11349/11352/11513/14705/18574 [newurl_path_link] => Odkaz na newurlCMS [iduzel] => 18574 [platne_od] => 06.09.2024 11:20:00 [zmeneno_cas] => 06.09.2024 11:21:40.276849 [zmeneno_uzivatel_jmeno] => Martin Klajmon [canonical_url] => [idvazba] => 22943 [cms_time] => 1752879652 [skupina_www] => Array ( ) [slovnik] => Array ( ) [poduzel] => stdClass Object ( [24689] => stdClass Object ( [nazev] => Applied thermodynamics [seo_title] => Applied thermodynamics [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => 0001~~8yguTYovyUjNL6qMT60oSC3KzE3NK4kvzk3MyQEA.jpg [ogobrazek] => [pozadi] => [obsah] =>Head of group
Prof. Květoslav Růžička, Ph.D.
b kvetoslav.ruzicka![]() vscht.cz
vscht.cz
e +420 22044 4116
d A131
Team members
Prof. Michal Fulem, PhD.
Vojtěch Štejfa, PhD.
Alex Mathers
Václav Pokorný
Research topic
Description of the vapor-liquid and vapor-solid equilibria for i) high-boiling substances classified as potential contaminants; ii) organometallic compounds used as MOVPE precursors; iii) biogenic compounds (terpenes, fragrances, H-bonding compounds, infochemicals). This includes
a) experimental determination of vapor pressure in low-pressure region (below 1 kPa);
b) calorimetric determination of heat capacities and enthalpies of phase transitions for solids and moderately volatile liquids (temperature range from 2 K to 600 K);
c)state of the art ab-initio calculations of ideal gas thermodynamic properties;
d) simultaneous correlation (SimCor) of vapor pressures and thermal derived quantities, which allows evaluation of vapor pressure (and vaporization/sublimation enthalpies) in the region of extremely low vapor pressures, where direct determination is not feasible;
e) Development, validation and application of ab initio computational methodologies for calculations of thermodynamic properties, phase diagrams and polymorphism of molecular crystals;
f) Molecular-dynamics simulations and calculations of thermodynamic and structural properties of ionic liquids.
Sublimation enthalpies obtained by SimCor are used as a benchmark for development of ab initio methodologies leading to reliable lattice energies and prediction of sublimation equilibria.
Calorimetry is used also for studying novel inorganic materials used for fabrication of semiconductors; polyaromatic hydrocarbons and bitumens; compounds of pharmaceutical interest
[urlnadstranka] => [poduzel] => stdClass Object ( [52967] => stdClass Object ( [nazev] => [barva_pozadi] => cervena [uslideru] => true [text] =>
Find more information about Laboratory of Applied Thermodynamics at
atductp.webnode.cz
[iduzel] => 52967 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => infobox [html] => [css] => [js] => [autonomni] => 0 ) ) ) [iduzel] => 24689 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/applied_thermodynamics [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) [78786] => stdClass Object ( [nazev] => Laboratory of photoredox catalysis [seo_title] => Laboratory of photoredox catalysis [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => 0001~~c8rMjU8vyi8tiDcCAA.jpg [vyska] => md [ogobrazek] => [pozadi] => [obsah] =>Head of group
Ing. Daniel Bím, Ph.D.
b daniel.bim![]() vscht.cz
vscht.cz
e 22044 4492
d B2-150b
Team members
Tereza Edlová
We are looking for new team members!
Research topic
In our lab, we combine experimental techniques with computational modeling tools to understand and solve challenging issues in transition-metal photoredox catalysis. Our approaches range from organic and inorganic synthesis, molecular spectroscopy, electrochemistry, photochemistry to quantum chemical methods, providing flexibility to tackle challenging problems from multiple angles. By manipulating the ligands of transition metal catalysts and reaction conditions, we aim to explain how the pre-catalysts are converted by light or electric potential into catalytically active species and understand the relationship between their electronic structure and reactivity, stability, and speciation. The emphasis is placed on understanding the reaction mechanisms to improve the state-of-the-art catalysts or to design new, more efficient and selective systems for applications in sustainable organic synthesis and energy research.
[urlnadstranka] => [poduzel] => stdClass Object ( [78787] => stdClass Object ( [nazev] => [barva_pozadi] => cervena [uslideru] => true [text] =>Find more information about the Laboratory of photoredox catalysis at
dbim-group.vscht.cz
[iduzel] => 78787 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => infobox [html] => [css] => [js] => [autonomni] => 0 ) ) ) [iduzel] => 78786 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/laboratory_of_photoredox_catalysis [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) [77934] => stdClass Object ( [nazev] => Laboratory of computational thermodynamics [seo_title] => Laboratory of computational thermodynamics [seo_desc] => [autor] => [autor_email] => [obsah] =>Head of group
doc. Ing. Ctirad Červinka, Ph.D.
b ctirad.cervinka@vscht.cz
e 22044 4485
d ZB4-06
X: @CervinkaGroup
Team members
Ing. Martin Klajmon, Ph.D.
Ing. Jan Ludík
Ing. Petr Touš
M.Sc. Reynaldo Geronia II
Research topic
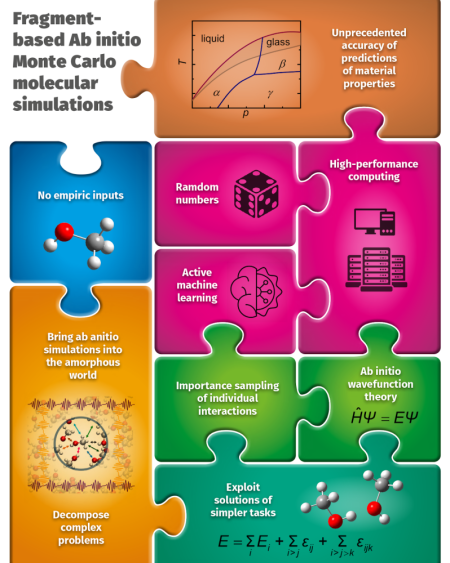
Find more information about Laboratory of computational thermodynamics at
https://sites.google.com/view/cervinkagroup/home
[iduzel] => 77937 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => infobox [html] => [css] => [js] => [autonomni] => 0 ) ) ) [iduzel] => 77934 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/laboratory_of_computational_thermodynamics [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [77933] => stdClass Object ( [nazev] => Laboratory of attosecond spectroscopy and dynamics of molecules [seo_title] => Laboratory of attosecond spectroscopy and dynamics of molecules [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => 0002~~MzIwMokPLstPyk9JjHcvSizIyExOzIlPTCouKUpMLokvMwIA.png [ogobrazek] => [pozadi] => [obsah] =>Head of group
Ing. Vít Svoboda, Dr. sc. ETH Zürich
b vit.svoboda![]() vscht.cz
vscht.cz
e 22044 4014
d Z B2-252
Team members
Tereza Pavlíčková
Eva Temelkova
We are looking for new team members
Research topic
Our group specializes in both experimental and theoretical studies of ultrafast processes in molecules and plasmonic nanoparticles. We study photoexcited dynamics by employing laser pulses lasting from hundreds to thousands of attoseconds. The generation of these pulses was awarded the Nobel Prize in Physics in 2023. Such short pulses enable us to observe the "flow" of electrons through molecules and thus understand the fundamental physical principles of the interaction between matter and radiation. With some exaggeration, we can say that our group develops "photon cameras" capable of shooting molecular movies of electrons dancing around atomic nuclei. From these electronic dynamics, we can determine, through appropriate experiments such as time-resolved photoelectron spectroscopy, the changes that occur after photoexcitation. One of our group's specialties is the study of chiral molecules. Specifically, we are interested in how the chirality of a molecule changes during a chemical reaction and how chiral molecules can manipulate electron spin. Understanding these fundamental principles might one day lead to the construction of a quantum transistor based on chiral molecules, which will serve in quantum computers operating at room temperature.
[urlnadstranka] => [poduzel] => Array ( ) [iduzel] => 77933 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/laboratory_of_attosecond_spectroscopy [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) [75563] => stdClass Object ( [nazev] => Theoretical Spectroscopy Research Group [seo_title] => Theoretical Spectroscopy Research Group [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => [ogobrazek] => [pozadi] => [obsah] =>Head of group
Eva Muchová
ORCID: 0000-0002-3768-6208
X: @EMuchova10
email: eva.muchova@vscht.cz
 Eva Muchová completed her PhD at Charles University under the supervision of Professor Pavel Hobza (Insitutute of Organic Chemistry and Biochemistry AS CR). From 2016 to 2023 she worked in the group of Professor Petr Slavíček at UCT as an assistant professor. In October 2019, she started a postdoctoral research position with Professor Benedetta Mannucci at University of Pisa, focusing on methods for the description of solvent for advanced X-ray spectroscopies. In January 2021, she moved back to UCT. Recently, she closely cooperates with Dr. Rebecca A. Ingle from UCL London, Prof. Olle Björneholm from Uppsala University or Dr. Milan Ončák from University of Innsbruck on multiple projects.
Eva Muchová completed her PhD at Charles University under the supervision of Professor Pavel Hobza (Insitutute of Organic Chemistry and Biochemistry AS CR). From 2016 to 2023 she worked in the group of Professor Petr Slavíček at UCT as an assistant professor. In October 2019, she started a postdoctoral research position with Professor Benedetta Mannucci at University of Pisa, focusing on methods for the description of solvent for advanced X-ray spectroscopies. In January 2021, she moved back to UCT. Recently, she closely cooperates with Dr. Rebecca A. Ingle from UCL London, Prof. Olle Björneholm from Uppsala University or Dr. Milan Ončák from University of Innsbruck on multiple projects.
News
A new paper in Nature Communications about the very first attoseconds of the birth of a hydrated electron.
Research interest
The research interests include theoretical modelling of optical and time-resolved X-ray spectroscopies, photoinduced processes in the solutions, theoretical photodynamics in realistic extended systems (liquids and solids), development of new approaches for ionization and electronically excited states.
[urlnadstranka] => [poduzel] => stdClass Object ( [75570] => stdClass Object ( [akce] => Theoretical Spectroscopy Research Group (Home) [objekt] => [odkaz] => /research/researchgroups/theoretical-x-ray-spectroscopy [iduzel] => 75570 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => [html] => [css] => [js] => [autonomni] => ) ) [75566] => stdClass Object ( [nazev] => People [seo_title] => People [seo_desc] => [autor] => [autor_email] => [perex] =>All B.Sc. and M.Sc. students are warmly welcome in A402! Please contact the group leader muchovae@vscht.cz
Alumni
Eva Didenko B.Sc. thesis (defended in 2023): Photochemistry of cyanine dyes
Marie Benešová B.Sc. thesis (defended in 2021): Chemistry and photochemistry of amoniacids on ice particles
Jan Cincibuch B.Sc. thesis (defended in 2021): Electrolytes in water and water in electrolytes, consultant
Radka Kittová B.Sc. thesis (defended in 2018): Aqueous droplets and ices in condensed phase: MD simulations of reversed micelles, consultant
Daria Victorovna Galaktionova B.Sc. thesis (defended in 2016): Optical Properties of Highly Absorbing Helquat Derivatives: Ab Initio Study, consultant
Michal BezekB.Sc. thesis (defended in 2015): Teoretical study of [2+2] photoaddition, consultant
Theoretical Resonant Auger Spectroscopy
At present, one of my main focus is the Auger resonance spectroscopy. Auger resonance spectra carry information of both the valence space of molecules (the most difficult region for chemists) and the unoccupied orbitals (which is important in photochemistry). However, modeling and interpretation of the spectra is much less developed than in photochemistry in the visible domain and it is developing a range of new techniques. In cooperation with experimentalits we study a small organic molecules and molecules with heteroatoms. The experiments are done at synchrotrons (for instance SOLEIL Paris). We are gradually beginning to understand the subtle details of the signals and the connection to chemistry and photochemistry of molecules.
Theoretical Photochemistry in Solid Phase
This project focuses on modelling solid-state photochemistry and developing tools to assist in their chemical analysis. Recently, much effort has been devoted to understanding and influencing the local environment of catalytic sites in solids so that we are able to achieve the desired properties and associated reactivity. Very promising systems are the so-called atomically dispersed catalysts in which metal atoms (ions of Co, Ni, Cu, Fe, etc.) or small clusters of metals are deposited on nanoparticles (MgO or ZnO, etc.), which have a wide range of practical applications from heterogeneous catalysis to sensing.
[ikona] => [obrazek] => [ogobrazek] => [pozadi] => [obsah] => [urlnadstranka] => /research/researchgroups/theoretical-x-ray-spectroscopy [iduzel] => 75567 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/theoretical-x-ray-spectroscopy/research [sablona] => stdClass Object ( [class] => stranka_submenu [html] => [css] => [js] => [autonomni] => 1 ) ) [75568] => stdClass Object ( [nazev] => Publications [seo_title] => Publications [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => [ogobrazek] => [pozadi] => [obsah] =>- Attosecond formation of charge-transfer-to-solvent states of aqueous ions probed using the core-hole-clock technique. E. Muchová*, G. Gopakumar, I. Unger, G. Öhrwall, D. Céolin, F. Trinter, I. Wilkinson, E. Chatzigeorgiou, P. Slavíček, U. Hergenhahn, B. Winter, C. Caleman, O. Björneholm, 2024, Nat. Comm. 15 (2024) 8903 https://www.nature.com/articles/s41467-024-52740-5
- Deconvolution of the X-ray absorption spectrum of trans-1,3-butadiene with resonant Auger spectroscopy D. M. P. Holland, J. Suchan, J. Janoš, C. Bacellar, L. Leroy, T. R. Barillot, L. Longetti, M. de Simone, C. Grazioli, M. Chergui, E. Muchová*, R. A. Ingle, 2024, https://doi.org/10.1039/D4CP00053F
- Oxygen Radicals Entrapped between MgO Nanocrystals: Formation, Spectroscopic Fingerprints, and Reactivity toward Water. T. Schwab, E. Muchová, K. Aicher, T. Berger, M. Ončák, O. Diwald, J. Chem. Phys. C 127 (2023) 23332–23339, https://pubs.acs.org/doi/full/10.1021/acs.jpcc.3c06091.
- Radiation damage by extensive local water ionization from two-step electron transfer mediated decay of solvated ions. G. Gopakumar, I. Unger, P. Slavíček, U. Hergenhahn, G. Öhrwall, S. Malerz, D. Céolin, F. Trinter, B. Winter, I. Wilkinson, C. Caleman, E. Muchová, O. Björneholm,Nat. Chem. 15 (2023) 1408–1414; associated contentResearch Briefing.
- Inner-Shell Photofragmentation of Adamantane (C10H16). S. Ganguly, M. Gisselbrecht, P. Eng-Johnsson, R. Feifel, S. Díaz-Tendero, E. Muchová, A. R. Milosavljević, P. Rousseau, S. Maclot, Molecules 28 (2023) 5510, https://doi.org/10.3390/molecules28145510.
- Spin–Vibronic Control of Intersystem Crossing in Iodine-Substituted Heptamethine Cyanines. R. Tovtik, E. Muchová, L. Štacková P. Slavíček, P. Klán, J. Org. Chem. 88 (2023) 6716–6728, doi: doi.org/10.1021/acs.joc.3c00005.
- Jahn–Teller effects in initial and final states: high-resolution X-ray absorption, photoelectron and Auger spectroscopy of allene. E. Muchová, D. Hollas, D. M. P. Holland, C. Bacellar, L. Leroy, T. R. Barillot, L. Longetti, M. Coreno, M. de Simone, C. Grazioli, M Chergui, R. A. Ingle, Phys. Chem. Chem. Phys, 25 (2023) 6733-6745, doi: 10.1039/d2cp05299.
- Observation of Intermolecular Coulombic Decay and Shake-up Satellites in Liquid Ammonia. H. C. Schewe, E. Muchová, M. Belina, T. Buttersack, D. Stemer, R. Seidel, S, Thuermer, P. Slavíček, B. Winter, Struct. Dynamics, 9 (2022) 044901, doi: 10.1063/4.0000151.
- Probing Aqueous Ions with Non-local Auger Relaxation. G. Gopakumar, E. Muchová, I. Unger, S. Malerz, F. Trinter, G. Öhrwall, F. Lipparini, B. Mennucci, D. Céolin, C. Caleman, I. Wilkinson, B. Winter, P. Slavíček, U. Hergenhahn, O. Björneholm, Phys. Chem. Chem. Phys., 24 (2022) 8661-8671, doi: 10.1039/D2CP00227B.
- Solvation energies of ions with ensemble cluster-continuum approach. L. Tomaník, E. Muchová, P. Slavíček, Phys. Chem. Chem. Phys. 22 (2020) 22357-22368, doi: 10.1039/D0CP02768E.
- Deciphering the Structure–Property Relations in Substituted Heptamethine Cyanines. L. Štacková, E. Muchová, M. Russo, P. Slavíček, P. Štacko, P. Klán, J. Org. Chem. 85 (2020) 9776–9790, doi: 10.1021/acs.joc.0c01104.
- Ionization energies in solution with the QM:QM approach. Z. Tóth, J. Kubečka, E. Muchová, P. Slavíček, Phys. Chem. Chem. Phys. 22 (2020) 10550-10560, doi: 10.1039/C9CP06154A.
- Do Water’s Electrons Care of Electrolytes? M. N. Pohl, E. Muchová, R. Seidel, H. Ali, Š. Sršeň, I. Wilkinson, B. Winter, P. Slavíček, Chem. Sci., 10 (2019) 848.
- Beyond Koopmans: Electron Binding Energies in Disordered Materials. E. Muchová, P. Slavíček, J. Phys. Condens. Matter. invited review, 31 (2019) 043001.
- Optimal Tuning of Range-Separated Hybrids for Solvated Molecules with TDDFT. M. Rubešová, E. Muchová, P. Slavíček, J. Chem. Theory Comput. 13 (2017) 4972.
- Molecular Dynamics and Metadynamics Simulations of [2 + 2] Photocycloaddition. E. Muchová, M. Bezek, J. Suchan, R. Cibulka, P. Slavíček, Int. J. Quantum Chem. 118 (2018) e25534.
- Observation of Electron-Transfer-Mediated Decay in Aqueous Solution. Unger, R. Seidel, S. Thürmer, M. N. Pohl, E. F. Aziz, L. S. Cederbaum, E. Muchová, P. Slavíček, B. Winter, N. V. Kryzhevoi, Nature Chemistry 9 (2017) 708.
- Photoswitching of Azobenzene-Based Reverse Micelles above and at Subzero Temperatures As Studied by NMR and Molecular Dynamics Simulations. L. Filipová, M. Kohagen, P. Štacko, E. Muchová, P. Slavíček, P. Klán, Langmuir 33 (2017) 2306.
- Modeling Liquid Photoemission Spectra: Path-Integral Molecular Dynamics Combined with Tuned Range-Separated Hybrid Functionals. D. Hollas, E. Muchová, P. Slavíček, J. Chem. Theory Comput. 12 (2016) 5009.
- Nature of CTAB/Water/Chloroform Reverse Micelles at Above- and Subzero Temperatures Studied by NMR and Molecular Dynamics Simulations. L. Klíčová, E. Muchová, P. Šebej, P. Slavíček, P. Klán, Langmuir 31 (2015) 8284.
- The Ice-Vapor Interface and the Melting Point of Ice Ih for the Polarizable POL3 Water Model. E. Muchová, I. Gladich, S. Picaud, P. N. M. Hoang, M. Roeselová, J. Phys. Chem. A 115 (2011), 5973.
- Glycine in an Electronically Excited State: Ab Initio Electronic Structure and Dynamical Calculations. E. Muchová, P. Slavíček, A.L. Sobolewski, P. Hobza, J. Phys. Chem. A, 111 (2007) 5259.
- Theoretical Study of Photoacidity of HCN: The Effect of Complexation with Water. E. Muchová, D. Nachtigallová, V. Špirko, P. Hobza, Phys. Chem. Chem. Phys. 8 (2006) 4866.
- Hydration-induced changes of structure and vibrational frequencies of methylphosphocholine studied as a model of biomembrane lipids. E. Mrázková, P. Hobza, M. Bohl, D. R. Gauger, W. Pohle, J. Phys. Chem. B 109 (2005) 15126.
- Lipid Hydration: Headgroup CH Moieties are involved in Water Binding. W. Pohle, D. R. Gauger, M. Bohl, E. Mrázková, P. Hobza, Biopolymers 74 (2004) 27.
- Hydration of Sulfo and Methyl Groups in Dimethyl Sulfoxide is accompanied by the Formation of Red-shifted Hydrogen Bonds and Improper Blue-shifted Hydrogen Bonds: An ab Initio Quantum Chemical Study. E. Mrázková, P. Hobza, J. Phys. Chem. A 107 (2003) 103.
Outreach publications
- Role kationtů v radiačním poškození. E. Muchová, J. Filgas, Aldebaran Bulletin 3 (2024).
- The Limits of the Periodic Table. E. Muchová, P. Slavíček, Chem. Listy 113 (2019) 198.
- In the Shadow of Electrons: Nuclear Quantum Effects in Chemistry. D. Hollas, E. Muchová, P. Slavíček, Chem. Listy 5 (2016) 349.
- Some Coul-de-sacs on the Way to Quantum Atom. E. Muchová, P. Slavíček, Chem. Listy 108 (2014) 638.
Book chapters
Nanodiamonds: Behavior in Biological Systems and Emerging Bioapplications I. Řehor, J. Šlegerová, J. Havlík, H. Raabová, J. Hývl, E. Muchová, P. Cígler Carbon Nanomaterials for Biomedical Applications, Book Series: Springer Series in Biomaterials Science and Engineering, 5 (2016) 319-361.
Nanodiamonds as Intracellular Probes for Imaging in Biology and Medicine J. Šlegerová, I. Řehoř, J. Havlík, H. Raabová, E. Muchová, P. Cígler Intracellular Delivery II: Fundamentals and Applications, Edited by: A. Prokop, Y. Iwasaki, A. Harada, A, Book Series: Fundamental Biomedical Technologies 7 (2014) 363-401.
[urlnadstranka] => /research/researchgroups/theoretical-x-ray-spectroscopy [iduzel] => 75568 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/theoretical-x-ray-spectroscopy/publications [sablona] => stdClass Object ( [class] => stranka_submenu [html] => [css] => [js] => [autonomni] => 1 ) ) [75569] => stdClass Object ( [nazev] => Grants [seo_title] => Grants [seo_desc] => [autor] => [autor_email] => [perex] =>Theoretical Photochemistry in Solid Phase
Česko-rakouské mobility 2023-2024, 8J23AT031
This project focuses on modelling solid-state photochemistry and developing tools to assist in their chemical analysis. Recently, much effort has been devoted to understanding and influencing the local environment of catalytic sites in solids so that we are able to achieve the desired properties and associated reactivity. Very promising systems are the so-called atomically dispersed catalysts in which metal atoms (ions of Co, Ni, Cu, Fe, etc.) or small clusters of metals are deposited on nanoparticles (MgO or ZnO, etc.), which have a wide range of practical applications from heterogeneous catalysis to sensing.
[ikona] => [obrazek] => [ogobrazek] => [pozadi] => [obsah] => [urlnadstranka] => /research/researchgroups/theoretical-x-ray-spectroscopy [iduzel] => 75569 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/theoretical-x-ray-spectroscopy/grants [sablona] => stdClass Object ( [class] => stranka_submenu [html] => [css] => [js] => [autonomni] => 1 ) ) [75564] => stdClass Object ( [obsah] => [iduzel] => 75564 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => slider [html] => [css] => [js] => [autonomni] => 0 ) ) ) [iduzel] => 75563 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/theoretical-x-ray-spectroscopy [sablona] => stdClass Object ( [class] => stranka_submenu [html] => [css] => [js] => [autonomni] => 1 ) ) [54862] => stdClass Object ( [nazev] => Biomolecular dynamics research group [seo_title] => Biomolecular dynamics research group [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => 0001~~KynPBwA.jpg [ogobrazek] => [pozadi] => [obsah] =>Head of group
doc. RNDr. Michal H. Kolář, Ph.D.
b kolarh@vscht.cz
e 22044 4257
d A402
Team members
Ing. Josef Cikhart
Mgr. Michaela Černeková
Ing. Hugo McGrath
MSc. Felipe C. Nepomuceno
Bc. Petr Linhart
Bc. Martin Mašek
Bc. Jakub Žváček
Aneta Hrádková
Petr Chalupský
Rudolf Kvasňovský
Arian A. Ott
Research topic
We are research group that lies on the borders of chemistry, biology, and physics. Our passion is to study how biomolecules move. And you can believe us, they move like Jagger! The zoo of biomolecules includes proteins, nucleic acids, membranes, polysacharides, and what all you know. Together, they are biopolymers: bio-, because they perform a biological function, and -polymers, because they consist of many simpler building blocks.
The function of biomolecules is tightly coupled to their structure and dynamics and we are keen to understand their underlying principles. Using large-scale computer simulation, database analyses, and statistical mechanics, we study details of biomolecular motion at atomistic resolution. Our favorite biomolecular beast is the ribosome (a cellular protein factory), but we also deal with smaller complexes and molecules. Although we do fundamental research, we believe that the problems we solve may help fight antibiotic resistance or design more efficient drugs against neurodegeneration, cancer or viral infections.
Figure: Bacterial ribosome cross section. The detail shows several conformations of a nascent peptide in the exit tunnel.
[urlnadstranka] => [poduzel] => stdClass Object ( [75601] => stdClass Object ( [nazev] => [barva_pozadi] => cervena [uslideru] => true [text] =>Find more information about the Biomolecular dynamics research group at
http://mhko.science
[iduzel] => 75601 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => infobox [html] => [css] => [js] => [autonomni] => 0 ) ) ) [iduzel] => 54862 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/biomolecular_dynamics_research_group [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) [24693] => stdClass Object ( [nazev] => Division of advanced nanospectroscopic techniques (DANTE) [seo_title] => Division of advanced nanospectroscopic techniques (DANTE) [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => [ogobrazek] => [pozadi] => [obsah] =>Head of group
Ing. Marcela Dendisová, PhD.
b marcela.dendisova![]() vscht.cz
vscht.cz
e +420 22044 3694
d AK07a
Team members
Pavel Matějka
Adéla Jeništová
Pavla Zaklová
Martin Král
Ladislav Lapčák
Karolína Salvadori
Matěj Kmetík
Ivan Kopal
Valerie Smeliková
Research topic
The research of the laboratory (DANTE) is focused on plasmonic nanomaterials and their use in the field of plasmonic-enhanced optical spectroscopies and near-field microscopy. We are interested in new preparation methods of metallic (Au, Ag and Cu) nanostructured systems applying different approaches (from classical chemical reduction, over electrochemical metal coating, vacuum sputtering, to “green” synthesis using natural products). We develop methodologies of surface-enhanced and tip-enhanced vibrational spectroscopic techniques (SEVS, TERS, SNIM) for characterization of nanostructured surfaces, studies of physico-chemical processes in single molecular layer on plasmonic antennas, e.g. in the presence of radiation (surface photocatalytic reactions). We study assembled systems in micron and sub-micron scale starting from model monolayers of small organic molecules, over biologically important compounds to real samples – tissues, extracts of natural materials. We use advanced instrumental equipment which allows us to combine optical spectroscopies with microscopic techniques from optical microscopy to nanoscopic techniques (AFM, STM etc.).
Raman microscope –Renishaw (UK) – a dispersion Raman spectrometer with three sources of irradiation 457, 633 and 785 nm, corresponding gratings of 1200, 1800 and 2400 l/mm are available for the lasers, the scattered radiation is detected by a CCD camera. A Leica optical microscope with four objectives with 5×, 20×, 50× and 100× magnification serves as the cuvette space.
Scanning probe microscope – Innova Bruker microscope allowing scanning in different modes: AFM, STM, ….
TERS – tip-enhanced Raman spectroscopy – this type of nano-resolution vibrational spectroscopy is enabled due to the connection of a Raman microscope with an Innova microscope. It is possible to measure in STM or Tunning fork modes, measure individual spectra (tip in, tip out) or spectral maps.
IR sSNOM - Near-field scanning microscope in the infrared region (Neaspec, Germany) – the device serves for nanoimaging the distribution of various functional groups absorbing IR radiation in the mid-infrared region. The radiation source is QCL lasers emitting radiation in three regions, the device is also equipped with an interferometer and a very sensitive MCT detector. The method is based on atomic force microscopy.
[urlnadstranka] => [iduzel] => 77001 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/dante/equipment [sablona] => stdClass Object ( [class] => stranka_ikona [html] => [css] => [js] => [autonomni] => 1 ) ) [77003] => stdClass Object ( [nazev] => Research and Projects [seo_title] => Research and Projects [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => [ogobrazek] => [pozadi] => [obsah] =>New superhydrophobic perfluorinated nanomaterials for advanced micro and bio applications 21-23131J GACR; a role: members of the team
Study of the initial self-assembly processes during the formation of biomimetic anchor layers 20-08679S GACR; a role: co-investigator
Study of plasticization of polymeric membranes and thermodynamics of multicomponent sorption for the development of effective membrane separations 18-08389S; a role: members of the team
Nonoobjects for water decontamination TJ01000072 TACR; a role: investigator
Development of temperature-dependent measurement techniques of surface-enhanced vibrational spectroscopy for bioanalysis and pharmacy GAP206/11/0951; a role: investigator
2025
Kopal, I.; Švecová, M.; Král, M.; Michalcová, A.; Lapčák, L.; Matějka, P.; Dendisová, M. Exploring the variability of methylene blue´s surface-enhanced Raman scattering spectra – Impact of the experimental conditions (Plasmonic metal and excitation wavelength) and ongoing reactions (Reduction and photon-induced demethylation). Colloids and Surfaces A: Physicochemical and Engineering Aspects 2025, 709, 136187. DOI: https://doi.org/10.1016/j.colsurfa.2025.136187.
2024
Palounek, D.; Vala, M.; Bujak, Ł.; Kopal, I.; Jiříková, K.; Shaidiuk, Y.; Piliarik, M. Surpassing the Diffraction Limit in Label-Free Optical Microscopy. ACS Photonics 2024, 11 (10), 3907-3921. DOI: 10.1021/acsphotonics.4c00745.
Kmetík, M.; Kopal, I.; Král, M.; Dendisová, M. Characterization of Modified PVDF Membranes Using Fourier Transform Infrared and Raman Microscopy and Infrared Nanoimaging: Challenges and Advantages of Individual Methods. ACS Omega 2024, 9 (23), 24685-24694. DOI: https://doi.org/10.1021/acsomega.4c01197.
Smeliková, V.; Kopal, I.; Člupek, M.; Dendisová, M.; Švecová, M. Unveiling the Crucial Role of Chemical Enhancement in the SERS Analysis of Amphetamine–Metal Interactions on Gold and Silver Surfaces: Importance of Selective Amplification of the Narrow Interval of Vibrational Modes. Analytical Chemistry 2024, 96 (14), 5416-5427. DOI: https://doi.org/10.1021/acs.analchem.3c05189.
Salvadori, K.; Churý, M.; Budka, J.; Harvalík, J.; Matějka, P.; Šimková, L.; Lhoták, P. Chemoselective Electrochemical Cleavage of Sulfonimides as a Direct Way to Sulfonamides. The Journal of Organic Chemistry 2024, 89 (3), 1425-1437. DOI: https://doi.org/10.1021/acs.joc.3c01932.
Kral, M.; Dendisova, M.; Svoboda, J.; Cernescu, A.; Svecova, M.; Johnson, C. M.; Pop-Georgievski, O.; Matejka, P. Nano-FTIR spectroscopy of surface confluent polydopamine films – What is the role of deposition time and substrate material? Colloids and Surfaces B: Biointerfaces 2024, 235, 113769. DOI: https://doi.org/10.1016/j.colsurfb.2024.113769.
Kočiščáková, Z.; Král, M.; Jeništová, A. Detection of fragrances on the skin and study of their interaction using infrared and Raman spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2024, 308, 123698. DOI: https://doi.org/10.1016/j.saa.2023.123698.
Surina, A.; Salvadori, K.; Poupě, M.; Čejka, J.; Šimková, L.; Lhoták, P. Upper Rim-Bridged Calix[4]arenes via Cyclization of meta Alkynyl Intermediates with Diphenyl Diselenide. Molecules 2024, 29 (6), 1237 https://doi.org/10.3390/molecules29061237.
Kopal, I.; Švecová, M.; Jeřábek, V.; Palounek, D.; Čapková, T.; Michalcová, A.; Lapčák, L.; Matějka, P.; Dendisová, M. Laser-Induced Reactions of 4-Aminobenzenthiol Species Adsorbed on Ag, Au, and Cu Plasmonic Structures Followed by SERS Spectroscopy. The Role of Substrate and Excitation Energy – Surface-Complex Photochemistry and Plasmonic Catalysis. ACS Omega 2024, 9 (5), 6005-6017. DOI: https://doi.org/10.1021/acsomega.4c00121.
Hovorka, Š.; Hrdlička, Z.; Jeništová, A.; Švecová, M.; Izák, P.; Čížek, J.; Michalcová, A.; Hadravová, R.; Vopička, O. Impacts of ions on the plasticization of cellulose triacetate by fluorinated ionic liquids: Thermal properties, microscopy, Raman spectra, and sorption of pure enantiomers. Polymer 2024, 290, 126502. DOI: https://doi.org/10.1016/j.polymer.2023.126502.
2023
Kopal, I.; Švecová, M.; Plicka, M.; Dendisová, M. Time dependent investigation of copper colloids SERS-activity. Materials Today Communications 2023, 35, 105722. DOI: https://doi.org/10.1016/j.mtcomm.2023.105722.
Salvadori, K.; Onali, A.; Mathez, G.; Eigner, V.; Dendisová, M.; Matějka, P.; Mullerová, M.; Brancale, A.; Cuřínová, P. An Insight into Anion Extraction by Amphiphiles: Hydrophobic Microenvironments as a Requirement for the Extractant Selectivity. ACS Omega 2023, 8 (46), 44221-44228. DOI: https://doi.org/10.1021/acsomega.3c06767.
2021
Švecová, M.; Volochanskyi, O.; Dendisová, M.; Palounek, D.; Matějka, P. Immobilization of green-synthesized silver nanoparticles for micro- and nano-spectroscopic applications: What is the role of used short amino- and thio-linkers and immobilization procedure on the SERS spectra? Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2021, 247, 119142. DOI: https://doi.org/10.1016/j.saa.2020.119142.
Svoboda, J.; Král, M.; Dendisová, M.; Matějka, P.; Pop-Georgievski, O. Unraveling the influence of substrate on the growth rate, morphology and covalent structure of surface adherent polydopamine films. Colloids and Surfaces B: Biointerfaces 2021, 205, 111897. DOI: https://doi.org/10.1016/j.colsurfb.2021.111897.
Švecová, M.; Volochanskyi, O.; Král, M.; Dendisová, M.; Matějka, P. Advantages and drawbacks of the use of immobilized “green-synthesized” silver nanoparticles on gold nanolayer for near-field vibrational spectroscopic study of riboflavin. Applied Surface Science 2021, 557, 149832. DOI: https://doi.org/10.1016/j.apsusc.2021.149832.
Král, M.; Dendisová, M.; Matějka, P. Development and characterization of a novel reference sample for tip-enhanced Raman spectroscopy. Monatshefte für Chemie - Chemical Monthly 2021, 152 (9), 1119-1125. DOI: https://doi.org/10.1007/s00706-021-02808-5.
Kludský, M.; Dendisová, M.; Hrdlička, Z.; Jeništová, A.; Hovorka, Š.; Vopička, O. Nafion modified with simple bases and amino acid derivatives: Survey of physical properties and search for effective pervaporation membranes. Polymer Engineering & Science 2021, 61 (3), 891-905. DOI: https://doi.org/10.1002/pen.25638.
2020
Jeništová, A.; Loula, M.; Mestek, O.; Ulbrich, P.; Matějka, P. The effect of silver nanoparticles on the penetration properties of the skin and quantification of their permeation through skin barrier. Journal of Nanoparticle Research 2020, 22 (11), 332. https://doi.org/10.1007/s11051-020-05061-9.
2019
Dendisová, M.; Němečková, Z.; Člupek, M.; Prokopec, V. EC-SERS study of phenolic acids sorption behavior on Au, Ag and Cu substrates – Effect of applied potential and metal used. Applied Surface Science 2019, 470, 716-723. DOI: https://doi.org/10.1016/j.apsusc.2018.11.120.
Dendisová, M.; Palounek, D.; Švecová, M.; Prokopec, V. SERS study of fluorescent and non-fluorescent flavonoids: What is the role of excitation wavelength on SERS optical response? Chemical Papers 2019, 73 (12), 2945-2953. DOI: https://doi.org/10.1007/s11696-019-00757-2.
Skoupá, V.; Jeništová, A.; Setnička, V.; Matějka, P. Role of TiO2 Nanoparticles and UV Irradiation in the Enhancement of SERS Spectra To Improve Levamisole and Cocaine Detection on Au Substrates. Langmuir 2019, 35 (13), 4540-4547. https://doi.org/10.1021/acs.langmuir.9b00358.
2018
Švecová, M.; Ulbrich, P.; Dendisová, M.; Matějka, P. SERS study of riboflavin on green-synthesized silver nanoparticles prepared by reduction using different flavonoids: What is the role of flavonoid used? Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2018, 195, 236-245. DOI: https://doi.org/10.1016/j.saa.2018.01.083.
Dendisová, M.; Jeništová, A.; Parchaňská-Kokaislová, A.; Matějka, P.; Prokopec, V.; Švecová, M. The use of infrared spectroscopic techniques to characterize nanomaterials and nanostructures: A review. Analytica Chimica Acta 2018, 1031, 1-14. DOI: https://doi.org/10.1016/j.aca.2018.05.046.
Jeništová, A.; Halajová, L.; Dendisová, M.; Matějka, P. Study of interactions between Gallic Acid and Skin Surface using Infrared Spectroscopy. Vibrational Spectroscopy 2018, 97, 119-128. DOI: https://doi.org/10.1016/j.vibspec.2018.06.009.
2017
Jeništová, A.; Dendisová, M.; Matějka, P. Study of plasmonic nanoparticles interactions with skin layers by vibrational spectroscopy. European Journal of Pharmaceutics and Biopharmaceutics 2017, 116, 85-93. DOI: https://doi.org/10.1016/j.ejpb.2016.12.011.
[urlnadstranka] => [iduzel] => 76990 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/dante/publications [sablona] => stdClass Object ( [class] => stranka_ikona [html] => [css] => [js] => [autonomni] => 1 ) ) ) [iduzel] => 24693 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/dante [sablona] => stdClass Object ( [class] => stranka_submenu [html] => [css] => [js] => [autonomni] => 1 ) ) [24692] => stdClass Object ( [nazev] => Theoretical photodynamics research group [seo_title] => Theoretical photodynamics research group [seo_desc] => [autor] => [autor_email] => [perex] =>
Head of group
prof. RNDr. Petr Slavíček, Ph.D.
b petr.slavicek![]() vscht.cz
vscht.cz
e +420 22044 3687
d A135
Team members
Michal Belina
Jan Poštulka
Jan Polena
Jiří Janoš
Josef Filgas
Tomáš Jíra
Tomáš Ovad
Research topic
Laboratory of Theoretical Photodynamics aims at modeling interactions between photons and molecules. Due to advancement of laser technologies, light can be these days controlled in time, energy, or position with unprecedented precision. Our goal is to understand how we can control the molecules via the light. In our work we investigate a wide range of topics, e.g., biomolecular photostability, atmospheric photodynamical processes following the excitation of the molecules, interaction of molecules with high energy radiation or photoionization and charge-transfer reactions. At the same time, we aim to model molecules beyond the gas phase, investigating finite molecular aggregates or molecules in the condensed phase. Part of our work is also necessarily devoted to the development of new and more efficient techniques of molecular simulations. We develop new methods for simulating electronic spectra, new quantum chemical approaches or novel approaches for modeling solvent.
[urlnadstranka] => [poduzel] => stdClass Object ( [75867] => stdClass Object ( [nazev] => [barva_pozadi] => cervena [uslideru] => true [text] =>Find more information about the Theoretical potodynamics research group at
photox.vscht.cz
[iduzel] => 75867 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => infobox [html] => [css] => [js] => [autonomni] => 0 ) ) ) [iduzel] => 24692 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/theoretical_photodynamics [sablona] => stdClass Object ( [class] => stranka [html] => [css] => [js] => [autonomni] => 1 ) ) [24691] => stdClass Object ( [nazev] => Division of Interfacial Phenomena [seo_title] => Division of Interfacial Phenomena [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => 0001~~S8xJrchNzMnMSi0rzq4EAA.jpg [obsah] =>Head of group
Assoc. Prof. Alexandr Malijevský, Ph.D.
b alexandr.malijevsky![]() vscht.cz
vscht.cz
e +420 22044 4093
d A327
Research topic
We study structure and phase behaviour of fluids that are in contact with confining (solid) walls using methods of statistical mechanics. It is well know that in the case of a planar wall, the wall-gas interface can exhibit wetting transition at a particular subcritical temperature. Our primary concern is to investigate the role of a wall geometry in adsorption behaviour of generic fluid models and to predict possible novel surface phase transitions induced by the wall geometry. In particular, we wish to determine their order and, in the case of continuous transitions, to evaluate the pertinent critical exponents. These studies can also be extended for dynamical processes of non-equilibrium systems.
[poduzel] => Array ( ) [iduzel] => 24691 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/statistical_theory_inhomogeneous_fluids [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) [24690] => stdClass Object ( [nazev] => Laboratory of phase equilibria: Measurement and prediction of solubilities [seo_title] => Laboratory of phase equilibria: Measurement and prediction of solubilities [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => [ogobrazek] => [pozadi] => [obsah] =>Head of group
Assoc. Prof. Karel Řehák, PhD.
b karel.rehak![]() vscht.cz
vscht.cz
e +420 22044 4039
d AJ02
Team members
Alumni
Research topic
The group focus on experimental determination of solubilities of solids, liquids and gases and on computational treatment of data that involves correlations and prediction of solubilities by means of equations of state and other thermodynamic models. The laboratory is equipped with experimental apparatuses (usually of own design) for measurement of phase equilibria and analytical instruments (GC, UV-Vis spectrophotometer, KF-coulometer, vibration tube density meter). An apparatus for determination of vapour–liquid equilibrium (at low pressures), an apparatus for measurement of gas solubilities (0.1–5 MPa) and various experimental devices for determination of liquid–liquid and solid–liquid equilibria are operated in the laboratory.
Correlations and predictions of solubilities are based on utilization of models for the excess Gibbs energy (Wilson equation, NRTL, UNIQUAC, UNIFAC, NRTL-SAC) and equations of state of the PC-SAFT type. Computational treatments of data are usually done by self-made programs.
[urlnadstranka] => [poduzel] => Array ( ) [iduzel] => 24690 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/phase_equilibria_02 [sablona] => stdClass Object ( [class] => stranka_ikona [html] => [css] => [js] => [autonomni] => 1 ) ) [18613] => stdClass Object ( [nazev] => State behavior of aqueous solutions [seo_title] => State behavior of aqueous solutions [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => Cibulka_molecules_WEB_eng.jpg [obsah] =>Head of group
Assoc. Prof. Ivan Cibulka, CSc.
b ivan.cibulka![]() vscht.cz
vscht.cz
e +420 22044 4063, +420 22044 3782
d AI04
Research topic
Experimental study of density and speed of sound for highly diluted aqueous solutions of organic substances in wide ranges of temperature and pressure. Evaluation of quantities related to chemical potential. Prediction of properties of aqueous solutions of organic substances using group contribution methods.
[poduzel] => Array ( ) [iduzel] => 18613 [canonical_url] => //ufch.vscht.cz/research/researchgroups/aqueous_solution_properties [skupina_www] => Array ( ) [url] => /research/researchgroups/aqueous_solution_properties [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) [24688] => stdClass Object ( [nazev] => Laboratory of membrane separation processes [seo_title] => Laboratory of membrane separation processes [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => 0001~~CyrNTozPTc1NKkrMSwQA.jpg [ogobrazek] => [pozadi] => [obsah] =>Head of group
Prof. Karel Friess, Ph.D.
b karel.friess![]() vscht.cz
vscht.cz
e +420 22044 4029
d A137
Team members
Ondřej Vopička
Dániel Gardenö
Tereza-Markéta Durďáková
Jana Floreková
Josef Schneider
Jonatan Štercl
Saeed Jamali Ashtiani
Alumni
Marek Lanč
Research topic
The laboratory specializes in studying membrane processes for separating gases, vapors, and liquids. The group has a wide range of experimental equipment for studying permeation, sorption, and pervaporation of compounds in membranes. Furthermore, the equipment for preparing nanoparticles (MOF) and polymer or graphene oxide membranes or composite materials with carbon nanotubes is also available. In addition, the group is also focused on surface modification of membranes and modeling the transport of compounds in membranes.
[urlnadstranka] => [poduzel] => stdClass Object ( [75598] => stdClass Object ( [nazev] => [barva_pozadi] => cervena [uslideru] => true [text] =>
Find more information about Laboratory of Membrane Separation Processes at
www.membranegroup.cz
The laboratory also forms a larger collaborative structure with the group of Surface and Interface Phenomena
[iduzel] => 75598 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => infobox [html] => [css] => [js] => [autonomni] => 0 ) ) ) [iduzel] => 24688 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/laboratory_membrane_separation_processes [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) [23106] => stdClass Object ( [nazev] => Surface and interface phenomena [seo_title] => Surface and interface phenomena [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => bart.jpg [ogobrazek] => [pozadi] => [obsah] =>Head of group
Ing. Štěpán Hovorka, Ph.D.
b stepan.hovorka![]() vscht.cz
vscht.cz
e +420 22044 4163
d A401
Team members
Alumni
Lidmila Bartovská
Research topic
The group is focused on a theoretical and experimental study of phase interfaces (vapor-liquid, liquid-liquid and solid-liquid) and colloidal systems. The particular topics are
a) Experimental study of the concentration and temperature dependence of surface and interfacial tension leading to an extension of a database with physical properties (e.g. solutions of organic non-electrolytes in water and aqueous solutions of inorganic salts and denaturants of proteins are the subject of the study)
b) A creation of a correlation equation (based on a theoretical model) that enables a quantitative description of mutual relations among physical properties of mobile phase interfaces (surface and interfacial tension) and a bulk of liquid phase (activity coefficients of components in the solution)
c) Experimental study of wettability of solids by liquids focused on pharmaceutical topics
d) Collaboration with ICPF of the CAS and IMC of the CAS on a project aiming at a resolution of racemic mixtures to particular enantiomers
e) Experimental study of physical properties of polymer membranes immersed in liquid solutions. Permeability and diffusion coefficients (i.e. kinetics of mass transport through the membrane), overall sorption of solution in the membrane and preferential sorption of particular components (phase equilibrium) and swelling of the membrane are measured. This activity is an extension (no duplication) of a research of Laboratory of membrane separation processes of Dr. Friess that is focused on membranes touched by gases and vapors
f) Experimental study of solutions of surfactants
[urlnadstranka] => [poduzel] => stdClass Object ( [75600] => stdClass Object ( [nazev] => [barva_pozadi] => cervena [uslideru] => true [text] =>The laboratory also forms a larger collaborative structure with the Laboratory of membrane separation processes.
[iduzel] => 75600 [canonical_url] => [skupina_www] => Array ( ) [url] => [sablona] => stdClass Object ( [class] => infobox [html] => [css] => [js] => [autonomni] => 0 ) ) ) [iduzel] => 23106 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/surface_and_interface_phenomena [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) [23053] => stdClass Object ( [nazev] => Laboratory of experimental thermodynamics: Phase equilibria and properties of mixtures [seo_title] => Laboratory of experimental thermodynamics: Phase equilibria and properties of mixtures [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => graf-spojnicovy [obrazek] => [obsah] =>Head of the group
Assoc. Prof. Vladimír Dohnal, CSc.
b vladimir.dohnal![]() vscht.cz
vscht.cz
e +420 22044 4297
d A133
Team members
Research topic
The research group is engaged in experimental study of equilibria in multicomponent fluid-phase systems and related thermodynamic and thermophysical properties of dilute aqueous and non-aqueous solutions of organic substances. To this end, we employ a large variety of procedures of experimental thermodynamics including phase equilibria measurements (vapor-liquid, liquid-liquid, solid-liquid), calorimetric measurements (enthalpy of mixing, dissolution), volumetric measurements, and measurements of viscosity and optical properties. Currently, we focus mainly on technologically, interesting systems with ionic liquids (IL), deep eutectic solvents (DES) and dilute aqueous solutions of volatile organic compounds (VOC) environmental and sensoric significance. Long-term effort of the group is devoted to experimental method and procedure development. New methods for determination of limiting activity coefficients, selectivity of non-volatile separation agents, mixing enthalpies and solubilities belong to our achievements in this direction.
[urlnadstranka] => [poduzel] => Array ( ) [iduzel] => 23053 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/phase_equilibria_01 [sablona] => stdClass Object ( [class] => stranka_ikona [html] => [css] => [js] => [autonomni] => 1 ) ) [22134] => stdClass Object ( [nazev] => Computer modeling of complex systems [seo_title] => Computer modeling of complex systems [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => ComplexSystems_title-900x900.png [obsah] =>Head of the group
RNDr. Mgr. Jan Heyda, Ph.D
b jan.heyda@vscht.cz
e +420 22044 4297
d A133
Team members
Kuhan Chandru (junior researcher - ChemJets)
Jakub Polák
Daniel Ondo (experimental)
Adam Kovalčík (experimental)
Denis Zadražil
Martin Melčák
Emil Pavelka
Research topic
In terms of computer simulations, we study the behavior of biomolecules in aqueous solutions, their thermodynamics and kinetics, action of salts and osmolytes. Grounded in the knowledge of molecular interactions we can rationalize the stability of protein native state, determine the solubility, or quantify the electrophoretic mobility. Moreover, for fast qualitative description of polymer and protein thermodynamics, we can determine in silico key parameters, which are further applied in thermodynamic models. Our approach is based on defining system key properties, applying an simulation protocol of optimal sampling efficiency, and finally evaluate the target property via statistical analysis of simulation data.
Website of Laboratory of modeling of complex systems
link to own website (in English)
[urlnadstranka] => [poduzel] => Array ( ) [iduzel] => 22134 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/computer_modeling_complex_systems [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) [18612] => stdClass Object ( [nazev] => Prediction of physicochemical properties of pure compounds and mixtures [seo_title] => Prediction of physicochemical properties [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => atom [obrazek] => [obsah] =>Head of group
Assoc. Prof. Pavel Chuchvalec, CSc.
b pavel.chuchvalec![]() vscht.cz
vscht.cz
e +420 22044 4023
d AJ02
Research topic
Development of methods for calculation and estimation of physical-chemical properties on the base of characteristic constants of compounds. Experimental data collection, their verification and critical evaluation. Finding appropriate correlation relationships. Utilization of database structures and program means for the efficient storage and retrieval of this information.
[poduzel] => Array ( ) [iduzel] => 18612 [canonical_url] => //ufch.vscht.cz/research/researchgroups/properties_prediction [skupina_www] => Array ( ) [url] => /research/researchgroups/properties_prediction [sablona] => stdClass Object ( [class] => stranka_ikona [html] => [css] => [js] => [autonomni] => 1 ) ) [18576] => stdClass Object ( [nazev] => Statistical thermodynamics and molecular simulations [seo_title] => Statistical thermodynamics and molecular simulations [seo_desc] => [autor] => [autor_email] => [perex] => [ikona] => [obrazek] => dog.jpg [ogobrazek] => [pozadi] => [obsah] =>Head of the group
prof. RNDr. Jiří Kolafa, CSc.
b jiri.kolafa@vscht.cz
e +420 22044 4257
d A402
https://web.vscht.cz/~kolafaj
Team members
Research topic
We investigate systems of many molecules by theoretical as well as pseudoexperimental methods using classical statistical thermodynamics.
While applying theoretical approaches as diagrammatic techniques and integral equations, we make use of modern methods of computer algebra.
The pseudoexperiment includes molecular dynamics and Monte Carlo simulations. We study a range of systems, from lattice models (e.g., of ice) to simple molecular system (hard spheres) to atomistic models (water and ice, salts, solutions). We also develop simulation methodology and force fields.
[urlnadstranka] => [poduzel] => Array ( ) [iduzel] => 18576 [canonical_url] => [skupina_www] => Array ( ) [url] => /research/researchgroups/statistical_thermodynamics [sablona] => stdClass Object ( [class] => stranka_obrazek [html] => [css] => [js] => [autonomni] => 1 ) ) ) [sablona] => stdClass Object ( [class] => stranka_ikona [html] => [css] => [js] => [autonomni] => 1 ) [api_suffix] => )